Scientists use many different instruments to determine the quality of water, including Secchi disks, probes, nets, gauges and meters. Water quality is not just measured by direct sampling. Information can also be derived from aerial and satellite photographs, by observing the surrounding environment and by collecting organisms that live in the body of water.
Although you might not have access to the resources of a scientist, there are some simple tests you can perform to get an idea of the quality of a particular water body. Learn about some of the different water quality tests below.

Temperature
The temperature of water can affect it in many different ways. Some organisms prefer cool water, while some like it warm. Most aquatic organisms are cold-blooded. This means that the temperature of their bodies matches the temperature of their surroundings. Reactions that take place in their bodies, like photosynthesis and digestion, can be affected by temperature. It is also important to know that when the temperature goes up, water will hold more dissolved solids (like salt or sugar) but fewer dissolved gases (like oxygen). The opposite is true for colder water. Plants and algae that use photosynthesis prefer to live in warm water, where there is less dissolved oxygen. Generally, bacteria tend to grow more rapidly in warm waters. Colder water contains more oxygen, which is better for animals like fish and insect larvae.
Dissolved Oxygen (DO)
Oxygen is necessary for many aquatic species to survive. Testing for dissolved oxygen (DO) tells you how much oxygen is dissolved in water for fish and other organisms to breathe. Most healthy water bodies have high levels of DO. Certain water bodies, like swamps, naturally have low levels of DO in the water. Lots of organic debris (fallen leaves, sewage leak) can cause a decrease in DO concentration. In the process of decomposing the organic material, microorganisms use all the oxygen in water. How does oxygen get in water in the first place? Much of the oxygen in water comes from plants during photosynthesis and also from air as wind blows across the water’s surface.
pH (acidity)
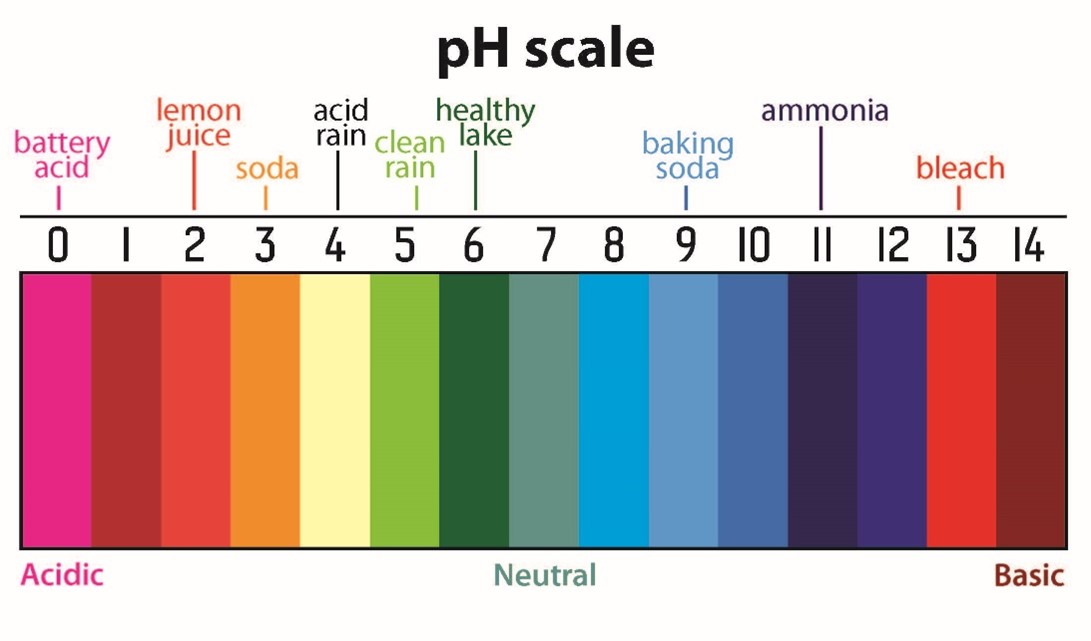
The potential of Hydrogen, also known as pH, is a measure of acidity and ranges from 0 (extremely acidic) to 14 (extremely basic) with 7 being neutral. Most water is in the range of 6.5–8.5. Let’s see some examples to compare pH values. Lemon juice has a pH of 3 — this makes it an acid. We all know how it feels to accidentally get lemon juice on a cut finger. Stronger acids have the ability to eat through solid objects if spilled. Liquid bleach has a pH of 11 — this makes it a base. Strong bases, just like acids, can burn your skin. Let’s think about why. Our bodies are made mostly of water. Water has a pH of 7. Things that are close to pH 7 work well with our bodies. The same holds true for aquatic organisms. If the water becomes too acidic or basic, it can kill them. Not all acids and bases are bad. Aspirin and tomatoes are acidic, while milk of magnesia and baking soda are both bases.
Turbidity
Turbidity refers to the clarity of water, or how clear it is. This determines how much light gets into the water and how deep it goes. Excess soil erosion, dissolved solids or excess growth of microorganisms can cause turbidity. All of these can block light. Without light, plants die. Fewer plants mean less dissolved oxygen. Dead plants also increase the organic debris, which microorganisms feed on. This will further reduce the dissolved oxygen. No dissolved oxygen means other aquatic life forms cannot live in the water.
Tips for Water Quality Monitoring
When testing the water quality parameters listed above, keep these tips in mind:
- After collecting your data, make a note of the time of year, current weather conditions, cloud cover, air temperature and any other environmental observations that may affect your results.
- Conduct and record results for multiple rounds of testing for each water quality parameter.
- If testing a body of water repeatedly to observe changes over time, use a map or other method to mark your testing location and make sure you test in the same spot each time.
Get involved in the EarthEcho Water Challenge, an international program that runs annually from March 22 (the United Nations World Water Day) through December.